Berlin, 12.01.2022 – The causes for the development of cancer are manifold. A multidisciplinary research team led by Charité – Universitätsmedizin and Goethe University Frankfurt am Main has investigated genes that are altered in lymph node cancer and thus identified a key process in cancer development. The signalling pathway, which has now been elucidated in detail, controls the repair of genetic damage. As the scientists describe in the scientific journal Nature Communications*, the findings could open up a new therapeutic approach.
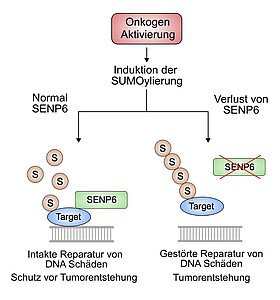
In cancer, various signalling pathways of the cell are disturbed. These include the so-called SUMOylation, a targeted modification of proteins that changes their properties and thus decides, for example, on their lifespan or localisation in the cell. “In our study, we were able to identify a previously unknown cancer gene that regulates this central signalling pathway in tumour diseases and could thus represent a target for new therapies,” says Prof. Dr. Ulrich Keller, Director of the Medical Clinic with a focus on haematology, oncology and tumour immunology at the Charité Campus Benjamin Franklin. He also heads a research group at the Max Delbrück Center for Molecular Medicine in the Helmholtz Association (MDC)
In order to identify and characterise such central switching points, a team of scientists from the Charité and the Goethe University systematically searched for genes that are altered in lymphomas – i.e. lymph gland tumours. To do this, they used a so-called transposon system. With this system, genes can be randomly switched on and off in the mouse model by “jumping” sections of genetic material and their effect on tumour development can be studied. “In recent years, numerous large sequencing studies have precisely characterised the genome of tumour diseases and illustrated the complexity and heterogeneity of the underlying alterations using ‘molecular maps’. The fact that such deviations often only occur in small groups of patients makes it difficult to interpret their significance,” explains Dr Markus Schick, team leader and principal investigator at the Charité’s Department of Medicine with a focus on haematology, oncology and tumour immunology and first author of the study. “Our approach has now enabled us to uncover numerous previously unknown cancer genes – including the gene SENP6, which is lost in about one third of all patients with lymphoma. Based on this, we have elucidated its functional mechanism and developed a therapeutic strategy.”
Until now, it was not known what role the gene plays in cancer. The protein SENP6 encoded by it removes the SUMO modifications from other proteins in the cell and thus also controls their interactions with each other. The research team has now been able to prove that the targeted silencing of SENP6 leads to carcinogenesis, i.e. it is a tumour suppressor gene. In healthy cells, SENP6 has a central role in the repair of DNA damage. After loss of the gene, this function is impaired and thus damage accumulates in the genome, which ultimately contributes to the development of cancer.
However, tumour formation after loss of SENP6 could be effectively suppressed by inhibiting the DNA repair enzyme PARP with the help of drugs that are already approved for breast cancer therapy. Prof. Dr. Stefan Müller, whose research group at the Institute of Biochemistry II at Goethe University was involved in the functional characterisation of the SENP6 protein, makes it clear: “A key to the success of the project is the combination of the biochemical expertise in Frankfurt and the clinical expertise at the Charité in Berlin.”
“With our findings, we were thus able to establish SENP6 as a biomarker for treatment success with such PARP inhibitors. We are currently investigating in which other tumour diseases – besides lymphomas – the newly described mechanism contributes to carcinogenesis,” summarises Prof. Keller. “The goal of personalised medicine is treatments that are precisely tailored to individual patients. The next step is therefore clinical trials to test these inhibitors as a new specific treatment option for cancers characterised by the loss of SENP6. In addition, combination therapies present themselves here, which are still rarely used but hold enormous potential – especially if they are used based on the patient’s own tumour biology.”
* Schick M et al. Genetic alterations of the SUMO isopeptidase SENP6 drive lymphomagenesis and genetic instability in diffuse large B-cell lymphoma. Nat Commun (2022), DOI: 10.1038/s41467-021-27704-8.
Photo Credit: Zelluläre Signalwege in der Krebsentstehung © Charité | Markus Schick
Links:
Medizinische Klinik mit Schwerpunkt Hämatologie, Onkologie und Tumorimmunologie
