Charité study to detect brain infarcts in comatose persons
Berlin, 12.04.2022 – Subarachnoid haemorrhage, a specific form of brain haemorrhage, can lead to an ischaemic stroke days later. Researchers at Charité – Universitätsmedizin Berlin have now shown that massive electrochemical waves in the brain announce a stroke in such a situation. By electro diagnostically monitoring these waves, brain infarctions can be detected in time – especially in patients who are receiving intensive medical care due to bleeding and are in a coma. The findings could lay the foundation for new therapies and have now been published in the scientific journal Brain*.
Subarachnoid haemorrhage is a form of brain haemorrhage in which blood spreads over a large area between the skins surrounding the brain. This form of haemorrhagic stroke is a neurological emergency, which is why those affected need immediate intensive medical treatment. If brain cells are not damaged by a cerebral haemorrhage, but by an acute lack of blood supply to an area of the brain, we speak of ischaemic strokes. Subarachnoid haemorrhages can in turn lead to ischaemic strokes. More than half of the patients with severe subarachnoid haemorrhage develop such a stroke within the first two weeks after the haemorrhage.
Scientists at Charité have now identified a biomarker that indicates a high risk of impending stroke after a subarachnoid haemorrhage. “Especially in people who are in a coma and cannot provide any information about their condition, it is difficult to assess when a new cerebral infarction might develop,” explains Prof. Dr. Jens Dreier from the Centre for Stroke Research at Charité and first author of the publication. “In our study, we show that electrodiagnostic monitoring makes this point in time visible. In this way, therapy can be initiated in time, even in comatose patients, before it is too late.”
Together with his team, Prof. Dreier discovered the biomarker based on the so-called “spreading depolarisations”. These are massive electrochemical discharge waves caused by the toxic blood degradation products of the brain haemorrhage. The brain areas affected by this then need a lot of energy to return to normal. In a healthy brain, very brief depolarisations of nerve cells, i.e. changes in membrane voltage, are normal and are coupled with the blood supply. This means that the brain can widen the vessels accordingly and compensate for an increased energy demand with increased blood flow. However, if the massive, prolonged and pathological spreading depolarisations occur after a subarachnoid haemorrhage, additional signalling cascades between nerve cells and blood vessels may be disturbed, so that the nerve cell discharge triggers extreme constriction of the vessels. As a result, the nerve cells lack the energy to recharge. If they remain in this discharged state for too long, they eventually begin to die.
“However, one scientific finding of recent years is central,” emphasises Prof. Dreier: “The discharge wave is reversible to a certain extent. This means, therefore, that the nerve cells can also recover if the nerve tissue is supplied with blood and thus oxygen in time.”
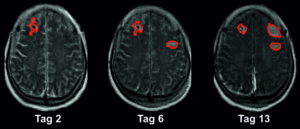
This is where the present clinical study, which was conducted at five different university hospitals, comes in. In order to precisely measure the spreading depolarisations, the researchers used electrocorticography, a method of modern neurointensive medicine for electrodiagnostic monitoring of brain waves. For this purpose, electrodes were implanted under the hard meninges of patients with subarachnoid haemorrhage when they were admitted to hospital. In addition, the researchers used imaging methods such as magnetic resonance imaging (MRI) and computer tomography (CT). They evaluated a total of about 1,000 images of the brain of 180 patients with subarachnoid haemorrhage. In this largest clinical study on spreading depolarisations to date, they found that an average of 46 millilitres of brain tissue is lost in the early phase, i.e. already when the patients come to the clinic. Another 36 millilitres on average are damaged in the first two weeks while the patient is in intensive care.
“These 36 millilitres of brain tissue could in principle be saved,” explains Prof. Dreier. “We can electro diagnostically detect the development of the brain infarcts at a stage where the changes are still reversible and modifiable. The observation of spreading depolarisations can thus be used as a biomarker in real time. In a way, it replaces the exchange with patients who cannot express their limitations and suffering because they are unconscious. In this way, we can identify those who are at risk of another stroke and initiate appropriate therapeutic measures at an early stage. On the other hand, people who are not threatened with another cerebral infarction do not receive any additional medication. Potential side effects can be avoided in this way.”
This procedure corresponds to the approach of precision medicine, in which the therapy is specifically tailored to the individual. In the future, the researchers would like to further test the monitoring of spreading depolarisations as an early warning system and ideally establish it in everyday clinical practice in order to continuously improve the treatment options for strokes. Artificial intelligence methods will play a major role here in order to analyse the electrodiagnostic data automatically and thus alert intensive care staff in real time if the brain tissue of the unconscious patient is in a threatening situation.
*Three J et al. Spreading depolarisations in ischaemia after subarachnoid haemorrhage, a diagnostic phase III study. Brain (2022). doi: 10.1093/brain/awab457
